Pfizer, US ink $5.29B deal for possible COVID-19 treatment
Associated Press
November 19, 2021

NEW YORK — The U.S. government will pay drugmaker Pfizer $5.29 billion for 10 million treatment courses of its potential COVID-19 treatment if regulators approve it.
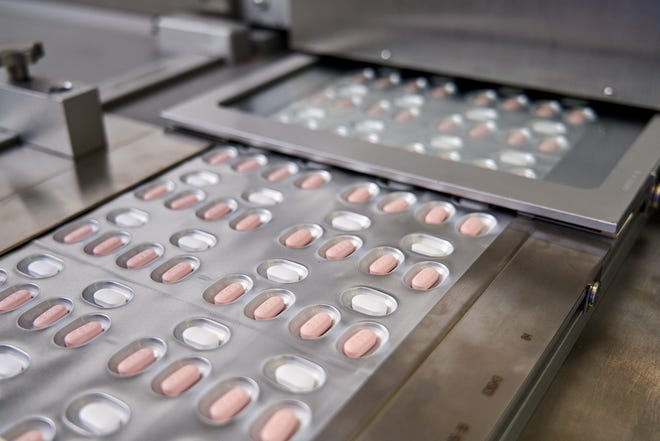
Pfizer asked the Food and Drug Administration on Tuesday to authorize the experimental pill, which has been shown to significantly cut the rate of hospitalizations and deaths among people with coronavirus infections.
Drugmaker Pfizer said Tuesday, Nov. 16, 2021, it is submitting its experimental COVID-19 pill for U.S. authorization, setting the stage for a likely launch in coming weeks.
The FDA is already reviewing a competing pill from Merck and will hold a public meeting on it later this month.
The price for Pfizer’s potential treatment amounts to about $529 per course. The U.S. has already agreed to pay roughly $700 per course of Merck’s drug for about 1.7 million treatments.
Pfizer said Thursday the price being paid by the U.S. government reflects the high number of treatment courses purchased through 2022.
Pfizer reported earlier this month that its pill cut hospitalizations and deaths by 89% among high-risk adults who had early symptoms of COVID-19. The company studied its pill in people who were unvaccinated and faced the worst risks from the virus due to age or health problems, such as obesity.
No comments:
Post a Comment